|
|
|
|
|
|
|
Dear Reader,
Following the shutdown of the facility in Spring 2020 due to the Covid-19 pandemic, ScopeM operation and activities, including users’ research projects, practical training, lectures… could, fortunately, be resumed in early Summer. I hereby want to thank all users, researchers and ScopeM staff for their commitment and flexibility. This enabled us to re-start and maintain an activity at a high level, despite the exceptional situation and necessity to comply with strict sanitary measures.
During the past 12 months, numerous new instruments were installed and are now available to the research community:
Ga-based FIBSEM – Helios 5 UX • Zeiss Inverted Lattice Lightsheet 7 – LLS • Confocal Raman – inVia • Fast Wide-Field Microscope – Ti2 • Stimulated Raman Scattering upgrade for SP8 • High-speed camera • Fluorescence Stereomicroscope – MZ 10F • Digital Microscope – DVM6 • Automated Freeze Substitution System – EM AFS2
A subset of those instruments is further described in this newsletter, with as well two contributions on i) recent results from a user’s project dealing with cryo-electron tomography and ii) a publication on reproducible image handling and analysis with practical recommendations for researchers and editors.
Furthermore, I am pleased to inform you that the decision was taken to simplify the fee system and that the applicable rates for EU projects have been set equal to the lower rates of SNSF.
I hope you enjoy this issue of ScopeM newsletter and wish you much success in your further research, teaching and projects.
Best regards,
Nicolas Blanc, PhD
Managing Director, ScopeM |
|
|
|
|
|
|
|
|
|
| Up & running: New Helios 5 UX FIBSEM |
A new state-of-the-art FIBSEM (Helios 5 UX by ThermoFisher Scientific, formerly FEI) has replaced the Zeiss NVision 40, which was decommissioned after more than 13 years of service at ScopeM. The new instrument was purchased in March 2020; installation started in June 2020; acceptance was signed in mid-August 2020. We have immediately started with training for staff and users, and the instrument is meanwhile heavily booked, mostly for user projects. Users of the former instrument could be smoothly transferred, also thanks to the scheduled overlap of about two months.
The new Helios 5 UX instrument shares the same platform as the existing Helios 600i. As a versatile analytical gallium FIBSEM operated at room temperature, it offers nearly all capabilities that were available on its predecessor except for an EBSD detector. The latter is available in the latest high-speed reincarnation on the analytical xenon Plasma-FIBSEM Tescan Fera.
The configuration of the new Helios 5 UX offers high-end performance by |
| • | | Elstar SEM column (ample detector options, notably three in-column detectors, high resolution imaging also at low kV by immersion mode, UC+ monochromator and deceleration, self-alignments) |
| • | | Phoenix FIB (new generation ion column, best low kV resolution, automated alignments) |
| • | | Easy-Lift EX Nanomanipulator (fully integrated, automated) |
| • | | MultiChem GIS (single Nozzle W, C, H2O, XeF2) |
| • | | Oxford Ultim Max 100 EDX detector (motorized, full SW suite) |
| • | | Kleindiek EBIC system (MM3A and EBIC amplifier) |
| • | | Kleindiek ACT and Nanolathe substages |
| • | | Micropolisher (low energy argon ion polishing system) |
| • | | CryoCan decontaminator |
| • | | Plasma cleaner |
|
The new multi-purpose height adjustable sample holder, aka "chair holder", see Fig. 1, allows users to easily mount their sample and set a safe stage height. In addition, it offers a 52° pre-tilted row-bar holder (takes up to 6 TEM grids). Both features are part of the scheme for fully automated TEM lamellae preparation by AutoTEM 5. Full automation means that the user only needs to define the position on the sample for extracting the chunk and the position on the grid for mounting the lamella. In addition to time savings for the operator, these automation solutions reduce the time needed to train new users and increase the reproducibility significantly. The possibility for unattended automatic operation overnight enables extended parameter optimization, which is expected to cater ultimate quality of the targeted preparation.
During the last 6 months, our priority was to get started and operate a running system. Besides of automation, several other exciting features are still to be explored and made use of. We are looking forward working with you on interesting research questions and samples.
Location: HPT C11
Contact: Dr. Joakim Reuteler or Dr. Peng Zeng |
 |
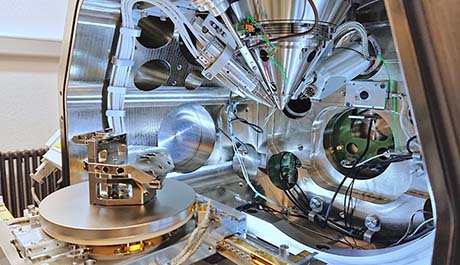 |
| Figure 1: View into the chamber of the Helios 5 UX with opened door. "Chair holder" mounted to the stage on the left, SEM column in the center, ICE detector in front next to the EasyLift manipulator needle, FIB column invisible behind, secondary electron detector in the back, next to it the CCD chamber camera. |
|
|
|
|
|
|
| Cryo-electron tomography of patient samples - how the protein uromodulin protects us against urinary tract infections |
Urinary tract infections are one of the most common bacterial infections. The vast majority of these infections are caused by uropathogenic E. coli, which use hair-like extensions, called pili, to bind to certain sugars found on epithelial cells lining the urinary tract. The most abundant protein in our urine, uromodulin, can counteract the adhesion of uropathogens. It therefor provides some protection against urinary tract infections, but the precise mechanism has been unclear. A recent publication in the journal Science by the Pilhofer and Glockshuber groups (IMBB) revealed the detailed mechanism of uromodulin’s protective function by using an integrative imaging approach.
Analyzing purified human uromodulin by cryo-electron tomography and subsequent subtomogram averaging showed that uromodulin forms stacked, fishbone-like filaments, consisting of a zigzag-shaped backbone and laterally protruding arms. Mapping the glycosylation sites with mass spectrometry together with biophysical assays demonstrated that uromodulin acts as a multivalent ligand for the pili of uropathogenic E. coli, by presenting specific sugars on its regularly spaced arms. Imaging uromodulin-pathogen interactions in vitro and directly in patient urine using light microscopy and cryo-electron tomography revealed that uromodulin can encapsulate uropathogens and mediates bacterial aggregation. By acting as a decoy, uromodulin subsequently prevents bacterial adhesion to the urinary epithelium and likely promotes pathogen clearance via micturition.
|
|
|
 |
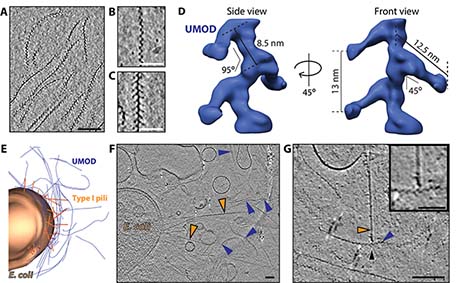 |
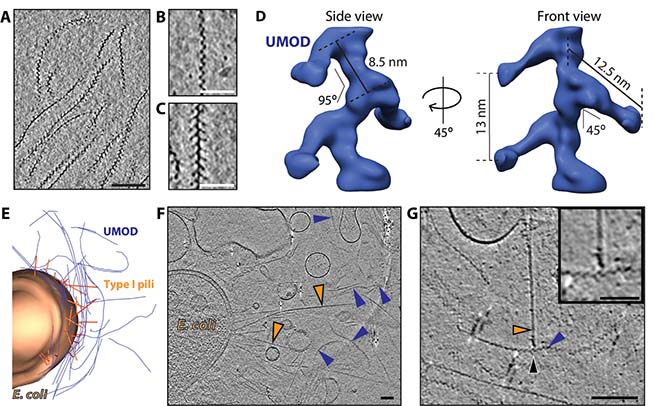
Figure 2: Cryo-electron tomography of human uromodulin filaments in urinary tract infections.
A Slice through a cryo-electron tomogram shows different orientations of purified uromodulin filaments. Bar, 100 nm. B and C show magnified views of most common orientations of uromodulin filaments. Bars, 50 nm. D Subtomogram average of uromodulin revealed a zigzag-shaped backbone with laterally protruding arms. Shown is an isosurface representation. E Three-dimensional segmentation of cryo-tomogram of E. coli cell (brown, pili in orange) incubated with uromodulin filaments (blue). Every imaged cell was surrounded by a mesh of uromodulin filaments. Bar, 100 nm F Cryo-electron tomography of urine from UTI patient showed bacterial cells with numerous pili (orange arrowheads) surrounded by several uromodulin filaments (blue arrowheads). G The cryo-tomograms of patient urine revealed several contact sides (black arrowheads) between uromodulin (blue arrowheads) and bacterial pili (orange arrowhead). Bar, 100 nm. Magnified view in box. Bar, 50 nm.
Cryo-electron tomography datasets were collected at ScopeM using Titan Krios with technical support from Mirek Peterek and Peter Tittmann. |
|
|
|
|
|
|
| Reproducible image handling and analysis |
Given the high volume of image data produced at ScopeM, we recently looked into potential pitfalls in image-analysis and how to avoid them.
The results of this inquiry we now share with you in an, open access, commentary in The EMBO Journal and its associated online comments section.
In it, we discuss how to avoid image-related misconduct and give practical recommendations for researchers and editors. For example, we show how to make your image-analysis code or image-data citeable using just GitHub and Zenodo. We also argue that code serves an additional role to automation, namely that of documenting the steps taken, ensuring that the analysis can be reproduced.
General data-management and the FAIR principles are not covered in depth in the commentary and we refer to the ETH library's knowledge, courses, and infrastructure on this topic, incl. requirements from the SNSF and EU funding agencies.
Our take home message is that, when it comes to image analysis, you and the rest of the scientific community will benefit greatly from a record it and report it approach. To learn what we mean by this and how to achieve it, read the paper and, if you have questions, come talk to us in the weekly Image Clinics.
Further resources |
|
|
 |
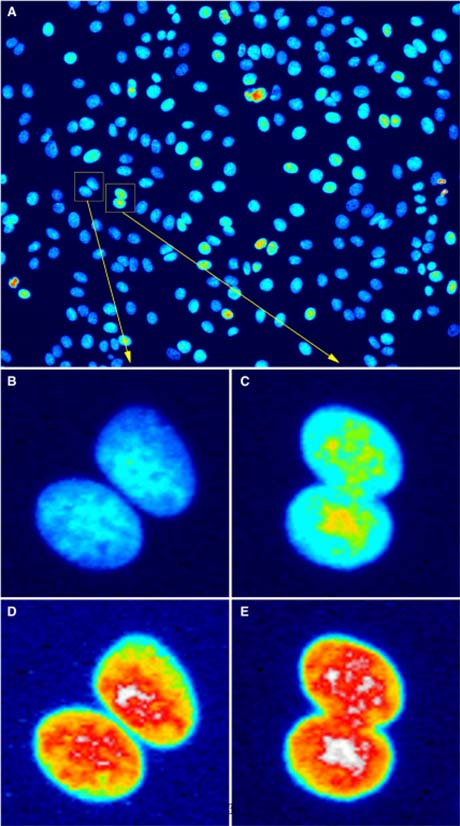 |
Figure 3: Bit depth conversion and normalization
|
|
|
|
|
|
|
New revolutionary lattice light sheet microscope at
ScopeM - Zeiss Lattice Lightsheet 7 |
The Zeiss lattice lightsheet is an inverted microscope platform focusing on fluorescence live cell imaging. Main benefits of this system are:
The Lattice Lightsheet 7 allows various experiment types: |
| • | | volume acquisition allowing fast acquisition speed/ high frame rates |
| • | | low photobleaching and low phototoxicity during acquisition |
| • | | easy, fast and stable light sheet acquisition settings |
| • | | easy sample mounting in standard sample carriers with no. 1.5 coverslip bottom (f.e. 35mm dishes, chamber slide or multi-well plates). |
|
| The Lattice Lightsheet 7 allows various experiment types: |
| • | | long-time recordings of single cells or smaller cell clusters |
| • | | fast acquisitions at high frame rates of small cell volumes (single cell) |
| • | | tracking experiments in one focal plane at highest frame rate |
|
| For further information see: ScopeM instrument website and the corresponding Zeiss product page. |
|
|
|
|
|
|
| New laser microdissection system – MMI-Nikon |
This microscope enables you to perform precise, non-contact laser based micromanipulation experiments. The MMI CellCut (from Molecular Machines & Industries ) facilitates precise and contamination-free dissection of cell clusters, single cells or subcellular compartments from various types of tissues including fresh frozen, paraffin embedded and archived slides, cytospins, smears and even living cells. The system is operated by it own, dedicated software.
Additionally, you are also able perform high-resolution wide-field imaging of fixed, stained and unstained samples and it is also possible to perform complex live cell imaging experiments. The "imaging functionality" is controlled by the NIS-Elements software (Nikon) which allows multichannel, multi-position, time-lapse imaging and complex experiments. |
|
|
|
|
|
|
| Stimulated Raman Scattering upgrade of the Leica SP8-CARS microscope - Taking the vibrational contrast to the next level |
Recently our Leica CARS microscope was upgraded with the Stimulated Raman Scattering (SRS) option. That is a contrasting technique that provides a rich, chemically specific image that arises directly from the endogenous molecules of the sample. No exogenous labels are required, in contrast to fluorescence microscopy. Both CARS and SRS enable label-free, up to video-rate imaging in living cells, tissues, and model organisms.
In contrast to CARS (that we have for a while now) SRS is a powerful, quantitative chemical imaging technique which overcomes any issue of autofluorescence stemming from the sample.
With this addition ScopeM can now provide to the users the full palette of Raman-based techniques. |
|
|
|
|
|
|
| New high-speed camera for a variety of imaging projects |
| ScopeM purchased a new 1MP high-speed camera that enables the use of very short exposure times (down to 200ns) and very high frequency acquisition (6400 frames/s with full resolution; up to 800,000 frame/s) to visualize fast moving objects. It can be directly mounted on the existing light microscopes in ScopeM (virtually on any one of them). For the use of the camera please contact Sung Sik Lee. |
|
|
|
|
|
|
| New Fluorescence Stereomicroscope MZ 10F |
| ScopeM offers newly an additional option of targeted sample preparation for correlative light and electron microscopy. The MZ10F is an up-grade of our cryo-ultramicrotome Leica EM FC6 Cryo and allows precise trimming of fluorescent samples under cryo-conditions or at room temperature. Fluorescent features can be targeted now directly for electron microscopy. Ultrathin sections for SEM area scans or TEM analysis can be acquired from a well-defined area of interest. The add-on allows to bridge functional light microscopy of biological samples and ultrastructural analysis by electron microscopy. |
 |
 |
Figure 4: Fluorescence Stereomicroscope MZ 10F
|
|
|
|
|
|
|
| Lower hourly rates for services provided by ScopeM in EU projects |
| Even though the hourly rates and costs eligible for reimbursement in EU projects for services provided by ETH technology platforms are – according to EU rules – higher than those for SNSF projects, the decision was taken to simplify the fee system of technology platforms and ScopeM: The applicable rates for EU projects have been set equal to the lower rates of SNSF. An overview of the hourly rates applicable for different costs categories can be found on our ScopeM website, whereas detailed information for each instrument is available to all registered ScopeM users in the booking system ppms. |
|
|
|
|